Research team of Prof. Ming Kuang from the First Affiliated Hospital of Sun Yat-sen University made breakthrough in m6A epigenetic mechanisms involved in hepatocellular carcinoma recurrence after radiofrequency ablation
Recently, Prof. Ming Kuang’s team from the Center of Hepatobiliary and Pancreatic Surgery, The First Affiliated Hospital of Sun Yat-sen University made a novel discovery of m6A epigenetic mechanisms involved in hepatocellular carcinoma recurrence after radiofrequency ablation (RFA). They published an original research paper entitled “Insufficient Radiofrequency Ablation Promotes Hepatocellular Carcinoma Metastasis through m6A mRNA Methylation Dependent Mechanism” online in Hepatology, which deciphered an m6A-dependent mechanism underlying how HCC cells response to sublethal heat stress through quick regulation of mRNA translation and promotes the recurrence of HCC after incomplete RFA (IRFA). The elucidation of such mechanism provides the strong scientific rationale for targeting RNA methylation machinery to prevent HCC recurrence in clinic RFA practice.
?
RFA is now recommended as one of the curative therapies for early stage HCC by guidelines of American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL). Despite of its prominent advantages as a minimally invasive treatment, the recurrence-free survival of HCC patients after RFA resembles that of surgery, with an approximate recurrence rate of 50% within 3 years. Treatment failure is mainly attributed to intrahepatic and distant metastasis after RFA, but the underlying mechanism remains elusive.
?
Given the implication of m6A modification in stress response and cancer progression, Prof. Ming Kuang’s team explored whether m6A machinery plays a pivotal role in HCC recurrence after sublethal heat treatment. Using IRFA HCC orthotopic mouse model, they detected higher level of m6A reader YTHDF1 in the sublethal-heat-exposed transitional zone close to the ablation center than that in the farther area. In addition, they validated the increased m6A modification and elevated YTHDF1 protein level in sublethal-heat-treated HCC cell lines, HCC patient-derived xenograft (PDX) mouse model and patients’ HCC tissues. Functionally, gain-/loss-of function assays showed that YTHDF1 promotes HCC cell viability and metastasis. Knockdown of YTHDF1 drastically restrains the tumor metastasis evoked by sublethal heat treatment in tail vein injection lung metastasis and orthotopic HCC mouse models. Mechanistically, they found that sublethal heat treatment increases EGFR m6A modification in the vicinity of 5’UTR region and promotes its binding with YTHDF1, which enhances the translation of EGFR mRNA. The sublethal heat induced up-regulation of EGFR level was further confirmed in IRFA HCC PDX mouse model and patients’ tissues. Combination of YTHDF1 silencing and EGFR inhibition suppressed the malignancies of HCC cells synergically. The in vitro and in vivo systems adopted by Prof. Ming Kuang’s team provide comprehensive tools for mechanism study and potential target for clinic intervention in HCC recurrence after IRFA. They demonstrate that IRFA promotes HCC recurrence in a m6A modification dependent manner, which serves as the first report presenting an epigenetic machinery in the process of HCC recurrence after IRFA.
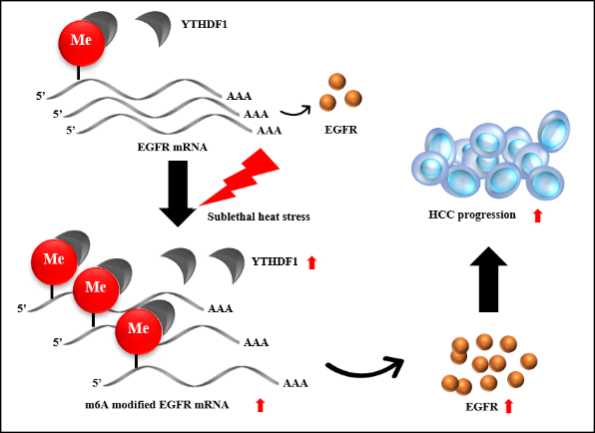
(Graphical abstract showing the m6A-YTHDF1-EGFR axis promotes HCC progression after IRFA)
Prof. Ming Kuang and Prof. Shuling Chen were the co-corresponding author. Doctor Tianhong Su, Manling Huang and Junbin Liao were the co-first author. This work is a new achievement of Prof. Ming Kuang’s team in the research field of RFA treatment in HCC.
?
Link to the paper: https://aasldpubs.onlinelibrary.wiley.com/doi/10.1002/hep.31766?
?

